Driving Innovation from Concept to Clinical Reality
Our Projects
At Astikay Medical, our projects reflect our passion for solving complex engineering challenges and bringing transformative medical technologies to life. From early-stage concepts to fully developed Class III implantable devices, we partner with our clients to design, prototype, and deliver solutions that advance patient care. Each project showcases our commitment to technical excellence, creative problem-solving, and seamless collaboration.

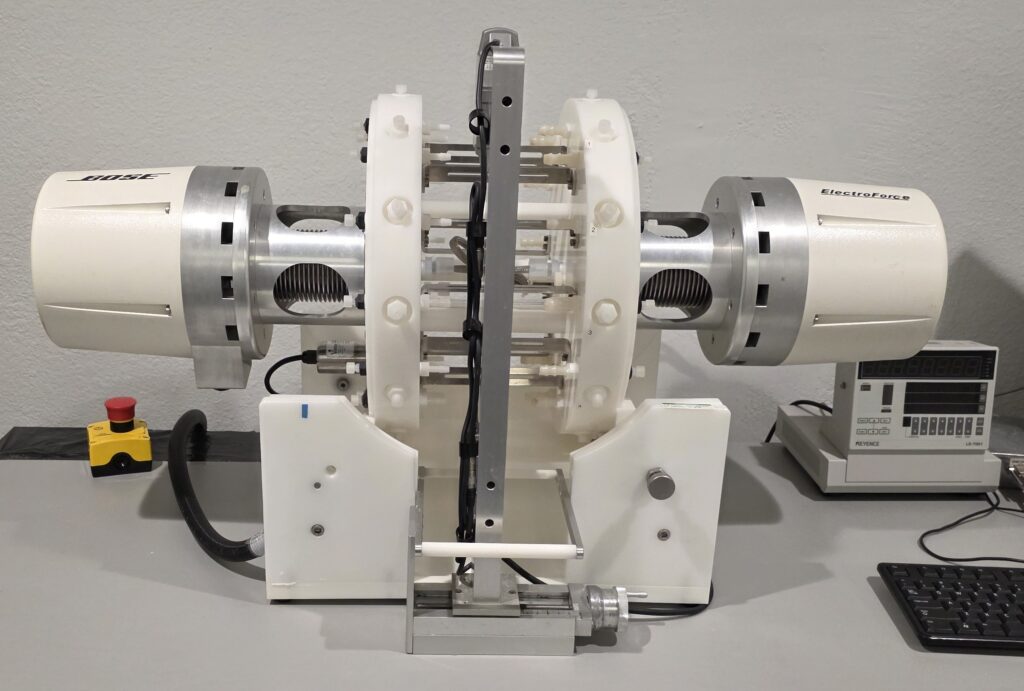

Mechanical & Fatigue Testing for FDA Approval
Overview
To support the FDA submission process, a comprehensive battery of mechanical and fatigue tests was conducted in strict accordance with regulatory requirements. These tests were essential to demonstrate the safety and efficacy of the medical device system.
Mechanical and Tensile Testing
Evaluations assessed the strength and durability of the delivery catheter and implant, providing critical data on their ability to withstand clinical use.
Simulated-Use Testing
Simulated-use testing was carried out to replicate conditions that mimic those experienced during actual procedures and ensure the devices maintained their performance and integrity throughout the expected lifecycle.
Long-Term Radial and Bending Fatigue Testing
Rigorous tests measured the devices’ resistance to repeated stress and deformation over time, crucial for long-term reliability and patient safety.
Regulatory Outcome
The successful completion of these comprehensive testing protocols directly contributed to achieving regulatory approval by our customers, verifying that the devices met all necessary standards for safety and performance.
Tissue Characterization Device Development
A low profile Guide Wire was engineered with integrated diagnostic probes designed to establish and access tissue types by precise measurement of their electrical properties. Custom electronic circuitry was developed under a range of simulated physiological conditions, in close collaboration with field experts, to ensure accurate determination of tissue composition. The system was further enhanced by unique software that incorporated machine learning algorithms, enabling continuous improvement in tissue characterization through Artificial Intelligence.

Custom Balloon Development
We offer Custom balloon development with providing guidance in design tailored to meet specific performance requirements. This process involves the careful selection and variation of balloon sizes, material properties, and functional characteristics to address the unique demands of various clinical applications.
Among the developed balloons were cryoballoons intended for the treatment of atrial fibrillation, specialized balloons supporting critical cardiovascular interventions with optimized performance, balloons for cheek augmentation providing effective solutions for facial cosmetic procedures, and high pressure spinal kyphoplasty balloons for improved outcomes in spinal surgery.

Neurovascular Device Development
Supported the design and development of multiple neurovascular devices for treating ischemic and hemorrhagic stroke. Collaborated with an international engineering team on design, prototyping, product requirements, and market analysis for flow diverters, aneurysm fillers, thrombectomy devices, and access catheters.

Drug Delivery Catheter with Temperature Sensing
A specialized drug delivery catheter was developed featuring integrated thermocouple to enable real-time temperature monitoring during procedures. This innovation ensures precise control over drug administration by continuously tracking temperature, which is essential for optimizing therapeutic outcomes and maintaining patient safety. The construction of the catheter was carefully engineered to maximize lubricity and meet rigorous performance standards, supporting smooth navigation and reliable drug delivery. The catheter was designed with an ergonomic handle to enhance user comfort and control throughout medical interventions.
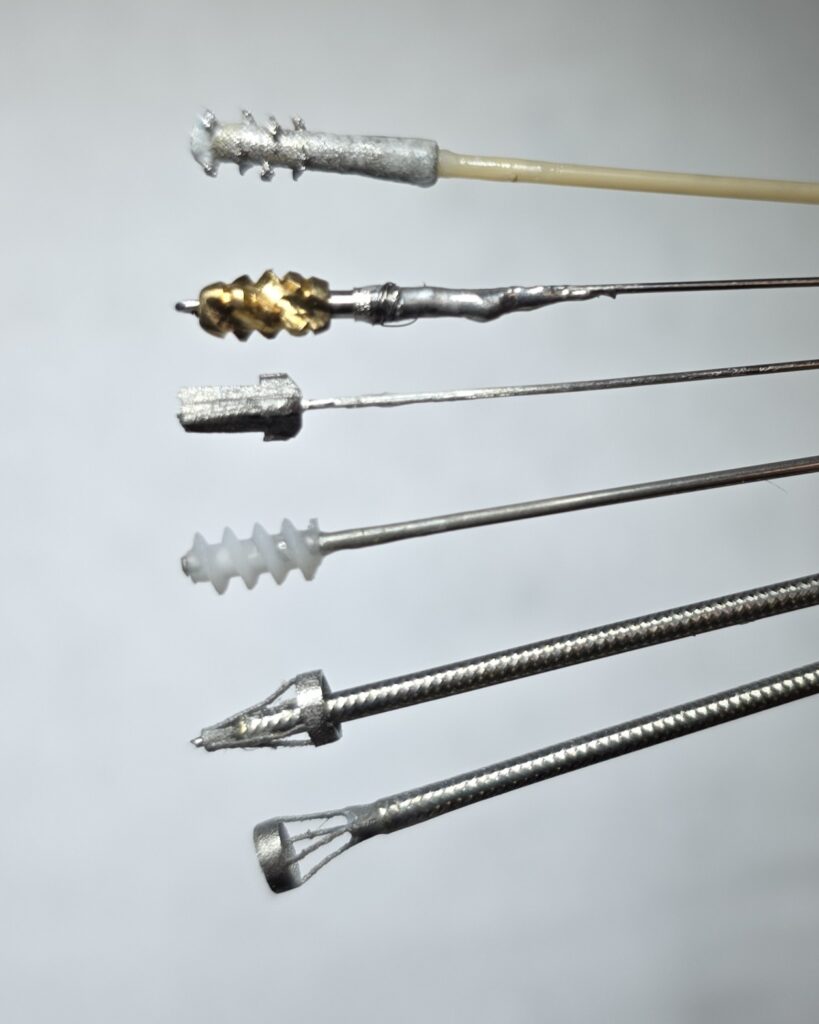
Pulmonary Embolism Thrombectomy Device
The design and development of a thrombectomy device specifically for the treatment of pulmonary embolism was undertaken with a clear focus on two primary goals: achieving effective clot removal and optimizing the device’s deliverability within the pulmonary vasculature.
To simulate clinical conditions and thoroughly evaluate device performance, blood clots were prepared in vitro. These in vitro models allowed for systematic testing and comparison of multiple design prototypes, ensuring that each option was rigorously assessed for its ability to remove clots efficiently.
A motorized handle was engineered to provide the high torque necessary for effective thrombectomy procedures. This feature was integral to the device’s function, supporting enhanced clot extraction capabilities while maintaining smooth operation within the pulmonary arteries.

Buccal Lipectomy
An innovative approach has been introduced in the field of cosmetic surgery for buccal lipectomy—a procedure aimed at removing fat from the lower cheek area to achieve a more contoured and chiseled facial appearance. Traditionally, this surgery involves making an incision from inside the mouth and utilizing standard surgical instruments such as scalpels to access and extract the buccal fat pads.
Astikay contributed to the advancement of this procedure by assisting in the development of a minimally invasive device that utilizes a gas-inflatable balloon. This device enables the removal of cheek fat through a small incision made on the outer surface of the cheek, as opposed to the conventional intraoral approach. The gas-inflatable balloon mechanism is designed to facilitate effective fat extraction with minimal tissue disruption, potentially reducing recovery time and improving procedural outcomes.
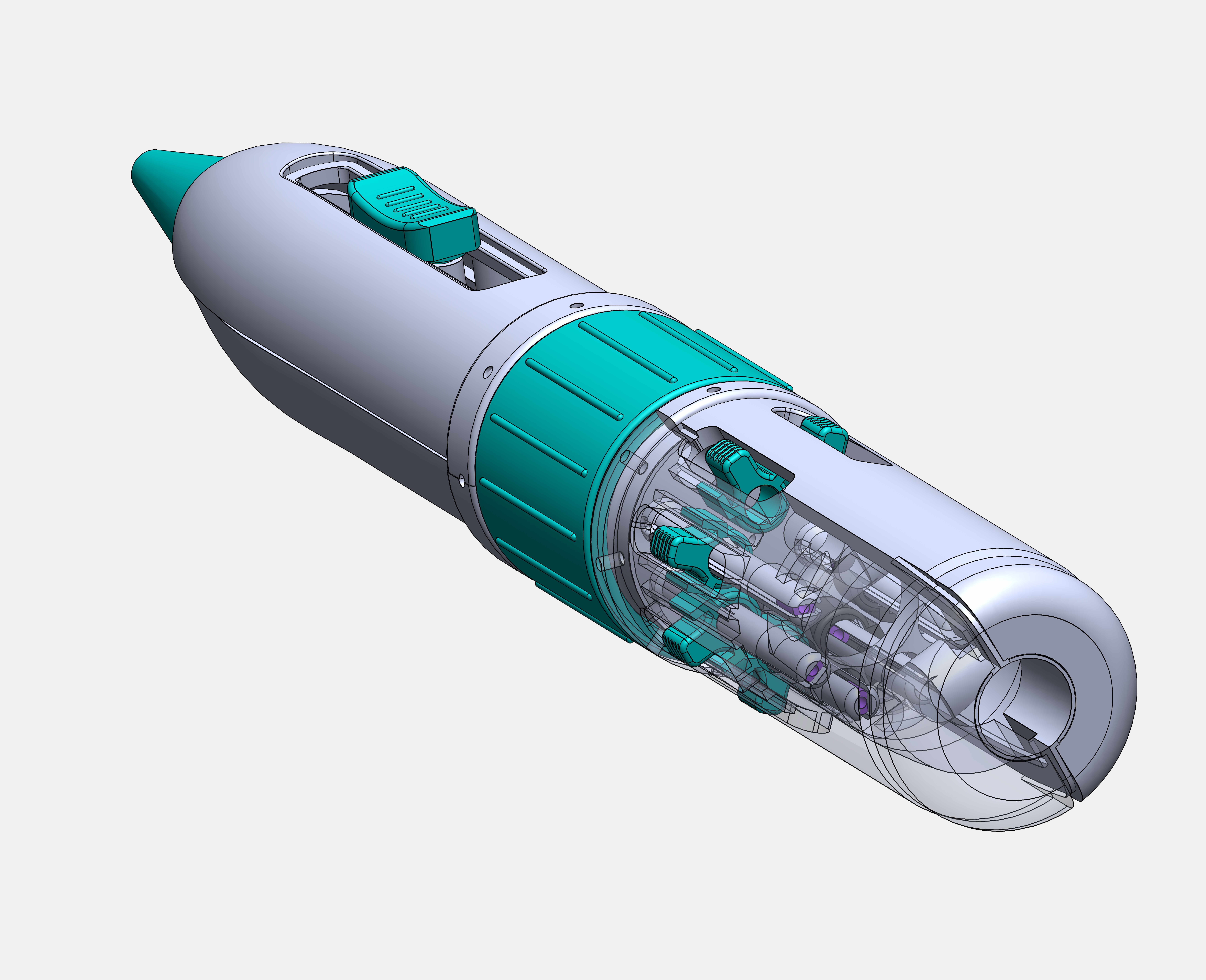
Handle Design – Structural Heart Device
The delivery of implant prosthesis requires most seamless surgical experience for varying degrees of complex procedures. We designed a multifunctional handle to delivery a TMVR implant that delivered twelve anchors around a positioning inflatable balloon. The material selection was crucial in preventing any compliance of the handle parts during procedure while our focus remained on key factors like ergonomics and maneuverability, surface texture for grip and precision in wet gloves, weight, sterilizability, biocompatibility, environmental conditions such as X-rays, along with regulations by FDA and ISO standards.
